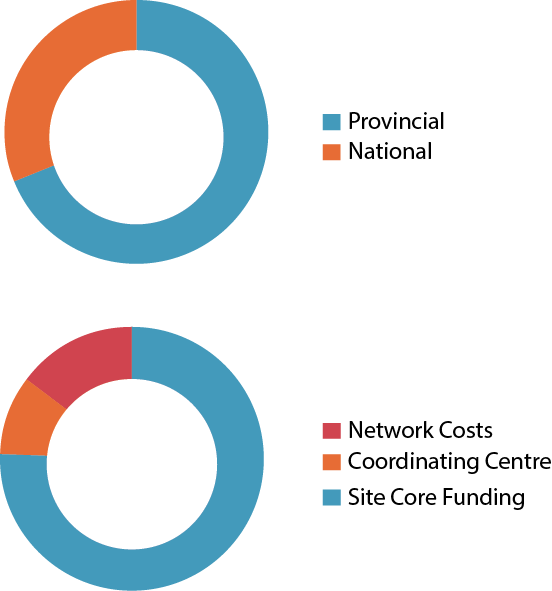Executive Message
On behalf of the Network, I am pleased to share highlights of 3CTN accomplishments over the past year. As member sites continued to recover the impacts of the COVID-19 pandemic on Canada’s academic cancer clinical trial (ACCT) environment, significant progress was made advancing the Network’s strategic priorities. In 2023, 3CTN sites across the country met trial performance targets and more than 40 new ACCTs were added to the 3CTN Portfolio – comparable to pre-pandemic years.
A major milestone was the establishment of our nationally recognized Equity, Diversity and Inclusion Framework to improve equitable access to trials for underrepresented populations. This builds on our leadership in developing CRAFT, the Canadian Remote Access Framework for Trials, which enables decentralized clinical trial (DCT) participation in our vast country where travel to a trial a site may be prohibitive.
Thanks to the leadership of Patient Representative members, and with industry funding we launched the new Patient Partner Learning Hub to strengthen patient engagement in trial conduct, strategic initiatives and advocacy.
We invite you to explore this report and the many examples that reflect 3CTN’s essential contributions to Canada’s ACCT ecosystem.

Stephen Sundquist, Executive Director
Recruitment

3833 Patients

20 Remote Access Patients

56 Adolescent & Young Adult Patients
3CTN EDI initiative aims to improve clinical trial participation among historically underrepresented populations
Clinical trials offer new and improved ways of diagnosing and treating those with cancer, giving patients access to potentially life-changing care options. However, many Canadian patients face systemic barriers – such as travel time and costs, as well as cultural and language challenges that limit their ability to participate in trials. When trial cohorts don’t reflect the diversity of the broader population, our understanding of how interventions will perform in real world settings is compromised.
To promote equity, diversity and inclusion (EDI) in clinical trials conduct, 3CTN launched an EDI Framework and Toolkit. These resources support member sites with best practices and links to helpful resources in specific areas of interest. Developed in collaboration with clinical research professionals, trial sponsors and people with lived experience, the resources reflect input from historically underrepresented groups, including older adults (70+), adolescents and young adults, rural residents, immigrants and newcomers to Canada, Indigenous peoples (First Nations, Métis and Inuit), racialized individuals, and members of the 2SLGBTQI+ community.
The EDI Framework and Toolkit were designed as living resources, enabling those involved to both benefit from and contribute to their continued development. 3CTN and its partners will regularly review and engage members and interested groups to ensure the materials remain current, reflect emerging evidence and incorporate evolving best practices.
“These resources provide us with a solid foundation on which to build a truly equitable, diverse and inclusive clinical trial ecosystem in Canada, I extend my heartfelt thanks to all of our partners who contributed to the development of these vital tools.” – Dr. Janet Dancey, 3CTN Scientific Director.

“For many people with cancer, clinical trials offer hope and a way to help shape the future of cancer care. There is no group of people that cancer does not touch, therefore it is critically important that this future is informed by a diverse range of experiences. I am proud of the collaborative effort to develop these new resources and am excited to see them being deployed across Canada.” – Michelle Audoin, Patient Partner
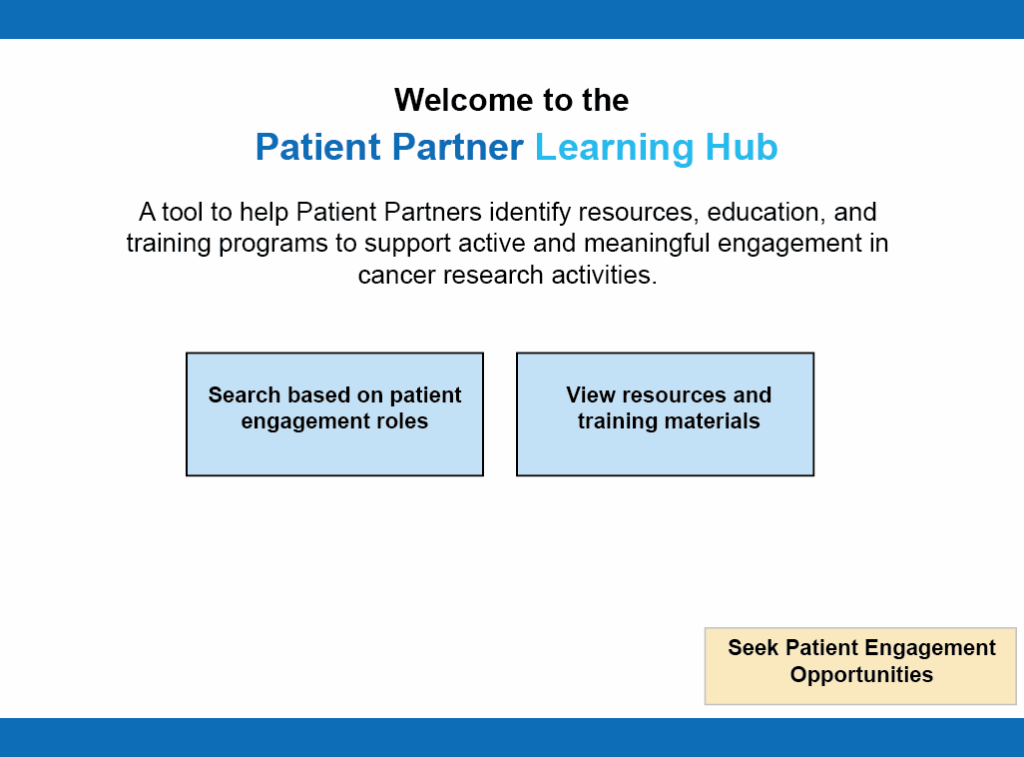
The Patient Partner Learning Hub: A Tool for Patient Partner Development
Embedding Patient Engagement (PE) to ensure patient values and perspectives are reflected in clinical trials conduct has been a core priority of 3CTN since its inception in 2014. Our community of Patient Representatives supports trial activities at local cancer centres and contributes to national priorities through participation in Network governance, working groups and projects.
To help sustain 3CTN’s PE framework, Patient Representatives identified a need for greater awareness and access to learning opportunities tailored to developing the knowledge and skills needed for meaningful patient engagement in research. With funding from Pfizer, 3CTN developed the Patient Partner Learning Hub, a structured, patient-centred platform for accessing educational materials related to clinical research and trials, as well as skill development for effective PE. The initiative was co-led by two patient partners, Judy Needham and Don Wood, who provided leadership and guiding input alongside a project team drawn from Network members.
The Learning Hub is organized around common knowledge areas and aspects of patient involvement. Its user-friendly interface supports individual learning goals by helping users find resources, training programs, and engagement opportunities. Researchers can also use the tool to support onboarding, orientation, and knowledge development for patient partners involved in site- or project-level activities needs.

“The Patient Partner Learning Annex has the potential to be a game changer for members of 3CTN’s Patient Representative Community as well as for others involved in patient engagement in research. They will benefit from a structured approach to developing clinical trials knowledge and competencies that will enhance the value of patient engagement.” – Don Wood, Patient Partner
Access.
CRAFT Toolkit
Across Canada, access to clinical trials is limited for many patients. Travel time and associated costs for rural and remote populations are prohibitive. With the development and implementation of CRAFT, the Network aims to provide equitable access to clinical trials for all Canadians, regardless of their location.
Network Achievements
3CTN and its partners developed two major tools to support inclusive and effective clinical trial conduct. The Patient Partner Learning Hub empowers Patient Partners to build skills and meaningful engagement in cancer research. The EDI Framework and Toolkit helps sites adopt best practices for engaging and including diverse populations in cancer clinical trials.
3CTN also continued to support in the rollout of the EDGE clinical trial management system across Canadian oncology clinical trials units. In 2023, the latest version, EDGE V3, was launched. It features a redesigned user interface and enhancements in areas such as finance and reporting. To support a smooth transition from V2 to V3, 3CTN led training sessions for site staff.
In collaboration with C17 and the pediatric network, 3CTN leverage EDGE’s collaborative capabilities and tools such as OPAL to develop a staff workload assessment tool. This tool OPAL scores with patient recruitment data from EDGE to assess and compare staff workload levels, with the goal of improving resource allocation, and preventing staff burnout.
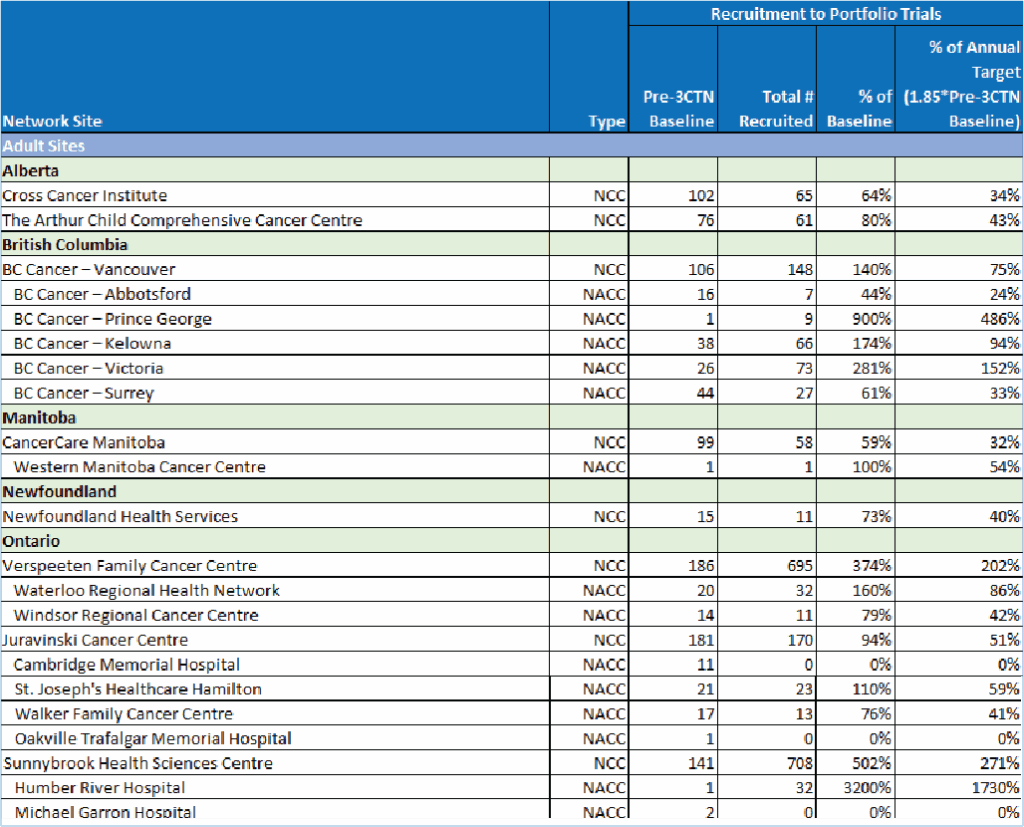
| Province | Number of Sites | Selected Network and site highlights |
|---|---|---|
| Alberta | 3 | – Trial unit staff participated in Diversity and Inclusion learning modules – New process implemented to track completion of staff training |
| British Columbia | 7 | – Participated in provincial EDI initiatives to improve EDI and best practices for cultural safety – Met local patient recruitment targets – Launched a hospital-wide EMR system |
| Manitoba | 3 | – Developed AYA strategy to pilot with an upcoming clinical trial – Recruited first patient within site target |
| Newfoundland | 2 | – Reviewed existing trial activation processes to identify opportunities for improvement. |
| Nova Scotia | 1 | – Implemented an improved trial activation process |
| Ontario | 29 | – Implemented an improved trial activation process, successfully activating a Portfolio trial within 120 days – Implemented a quality management system – Completed root cause analysis to identify factors impacting recruitment. – Developed workflow between primary and satellite site for CRAFT implementation |
| Quebec | 5 | – Implemented initiatives to improve communication and collaboration across oncology groups – Connected with site EDI lead to discuss how to improve access to trials |
| Saskatchewan | 1 | – Increased capacity to enable the opening of the first CNS trial in 10 years – Expanded access to clinical trials and recruited 4 remote access patients |
Outcomes.
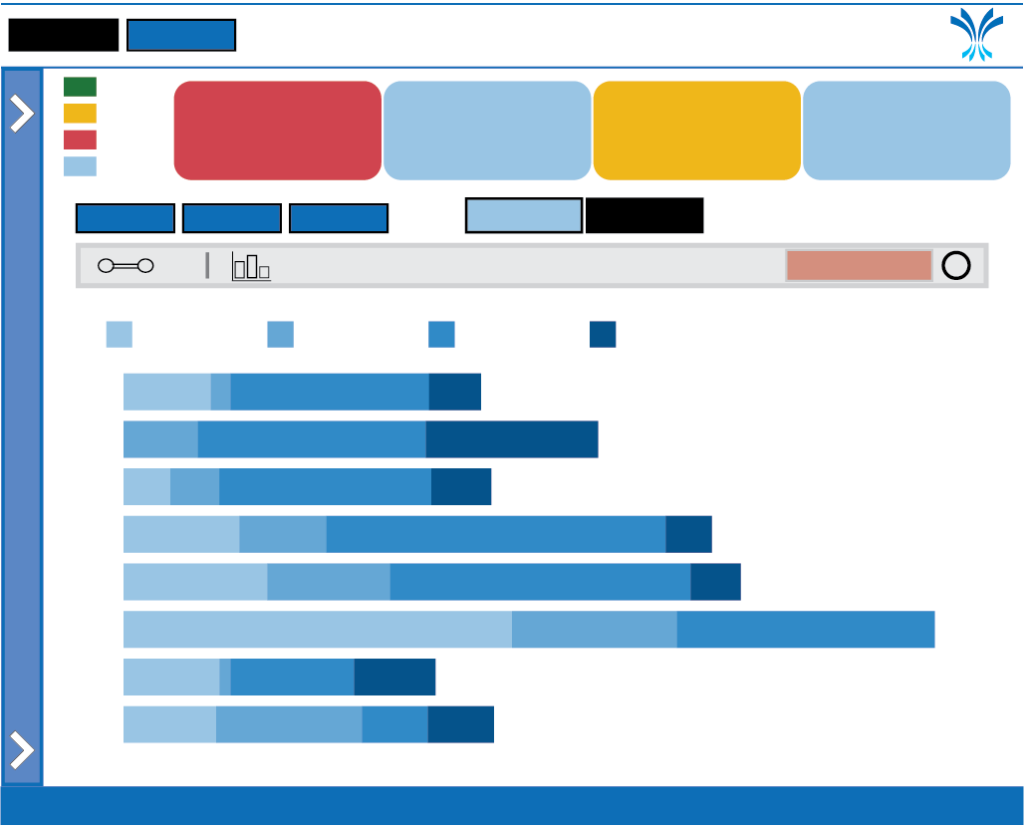
Network Performance Report
Compare and contrast network recruitment over the years. Explore top recruiting trials by quarter and fiscal year. Quickly see the networks performance to annual recruitment targets.
Portfolio Trial Applications

44 Trial Applications Reviewed

43 Trials Approved

4 Facilitated Peer Reviews Completed

6.24 Days Average
0 Days Median
Processing Time
Impact.
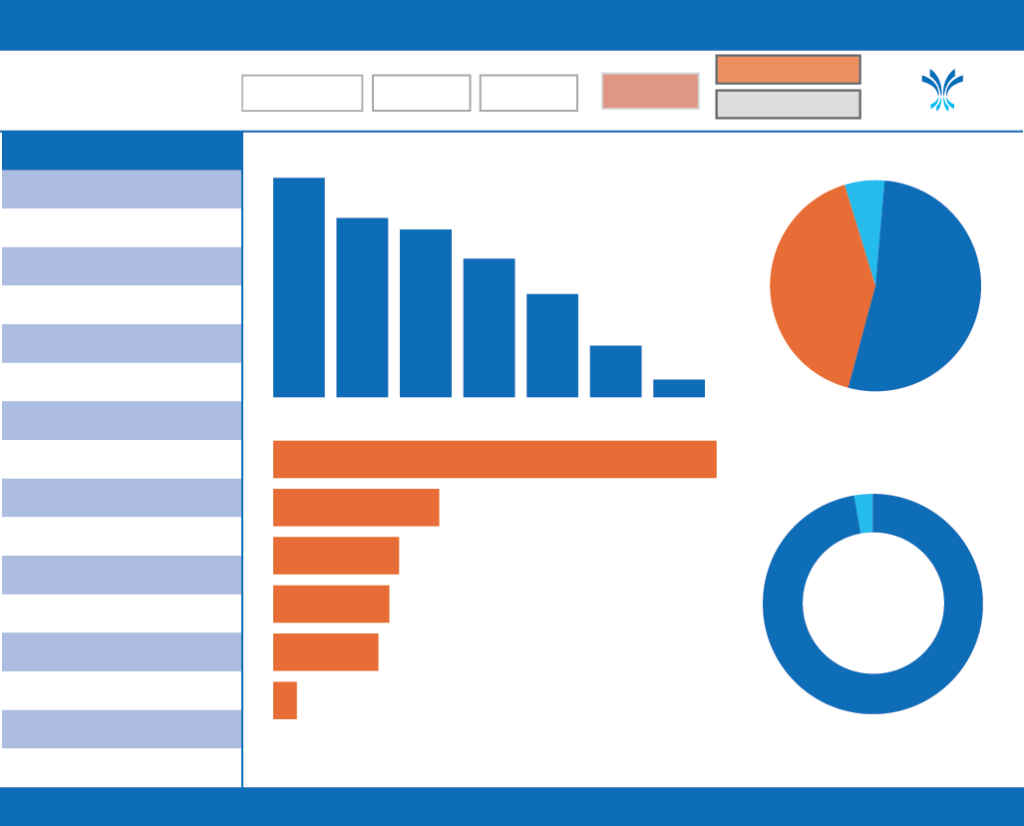
Portfolio Application Report
Compare and contrast 3CTN Portfolio trial applications totals and processing times over the years. See the current status of trials being reviewed. Find out which organizations submit the most Portfolio Applications.
Financials
Revenues
$ Amount in CAD
| Provincial | 1,650,000.00 |
| National | 3,646,375.00 |
| 5,296,375.00 |
Expenses
$ Amount in CAD
| Site Core Funding | 3,762,640.99 |
| Coordinating Centre | 485,705.10 |
| Network Costs | 730,292.24 |
| 4,978,638.33 |
Net (revenue less expenses)
317,736.67