Portfolio Assessment
Complexity Assessment
Once a trial has been approved for Portfolio status, the Coordinating Centre will review the trial and assign a trial complexity score and rating. The trial complexity rating will be inputted into the EDGE Clinical Trials Management System and be viewable on the 3CTN Portfolio website. Please refer to the Complexity Weighting Guidelines for more information and the complete complexity assessment table.
| Complexity Rating | Complexity Score |
|---|---|
| Low | 0-3 |
| Standard | 4-6 |
| High | 7+ |
| Other (e.g., Low / 2, Low / 10) | – |
Incentive Based Funding
3CTN’s funding model has been updated to include Incentive Based Funding (IBF) for the 2022-2027 business cycle. Please refer to the Incentive Based Funding Process for IBF factors and definitions.
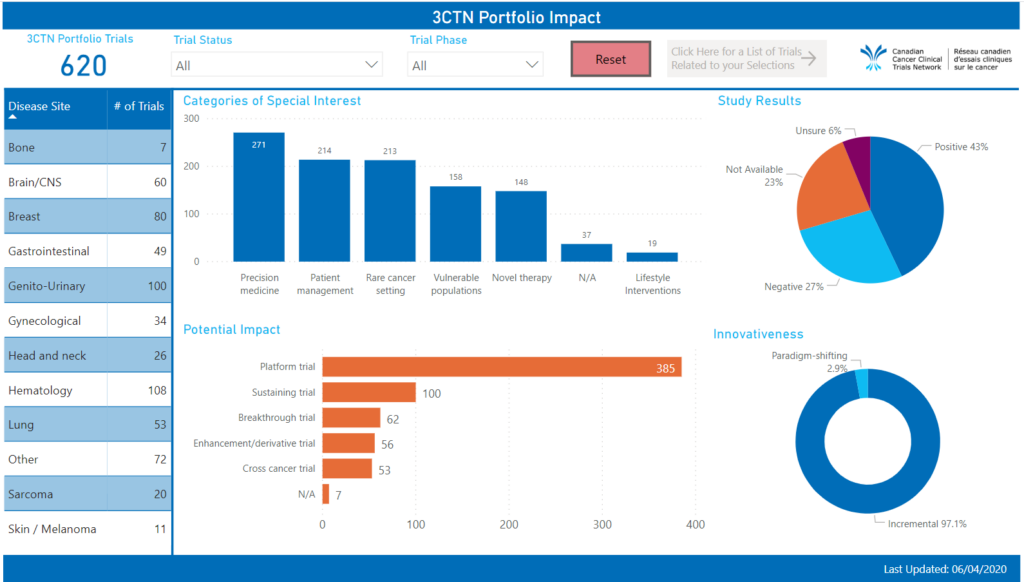
Portfolio Impact
Adapted from published literature, 3CTN created categories to describe the mix of trials that make up the Network’s Portfolio (full definitions). The customized framework developed with the Portfolio Committee’s input was derived from “The Importance of Doing Trials Right While Doing the Right Trials” paper (Dilts et. Al.) and can allow for analyses based on a standardized set of Study Criteria as well as defined Impact on Patient Population and Innovativeness (see below).
Trial categories of special interests: Novel Therapy, Rare Cancer Setting, Patient Management, Vulnerable Populations, Lifestyle Interventions, Precision Medicine
Potential Impact on Patient Population: Breakthrough Trial, Therapeutic Repurposing Trial, Biomarker-Guided Precision Trial, Treatment Optimization Trial, Cross Cancer Trial
Innovativeness of Trials: Incremental, Paradigm Shifting
Study Results: Positive, Negative
Results from periodic review will enable assessments of the different types of studies being undertaken at a given point, trends and changes over time as well as help articulate the overall impact of the Portfolio for funders and other stakeholders.
View an interactive report on the outcomes of 3CTN’s Portfolio Impact Analysis.