Portfolio Application
Portfolio Trial Eligibility
- An eligible trial must meet the following criteria:
- Interventional oncology trial;
- Academically sponsored (Clinical Trial Applications held by academic institution);
- Open to multiple Canadian sites;
- Funded independently of 3CTN;
- Peer reviewed by external reviewers.
- For additional details, see the Trial Eligibility Criteria and Guidelines.
How to Submit a Portfolio Application
To submit a trial for consideration for the 3CTN Portfolio, complete the Application Form and submit to info@3ctn.ca. Trials will be assessed according to the Portfolio Process Map. If your cancer centre is using EDGE please submit your application through EDGE following these steps.
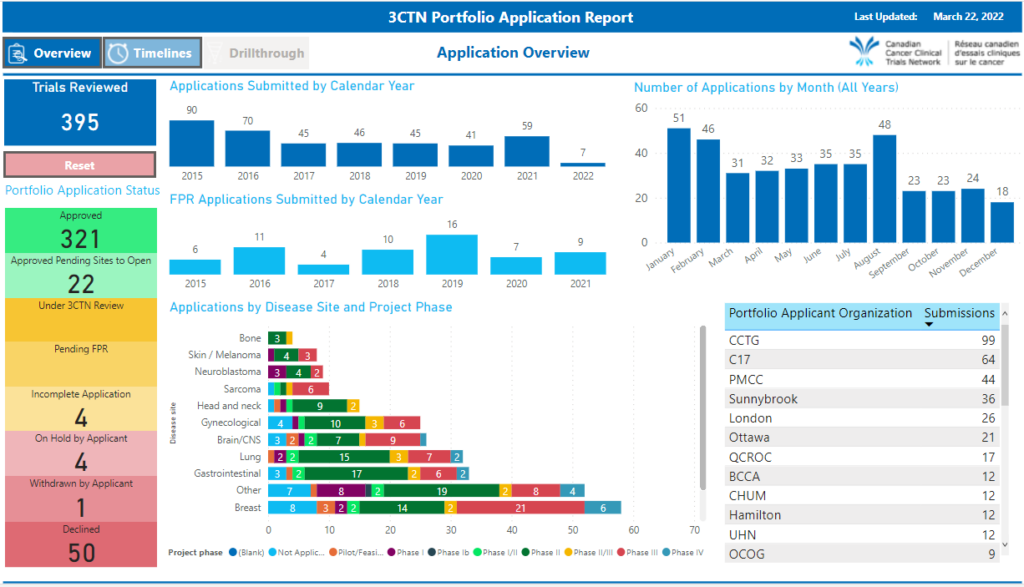
If you have recently submitted a trial for review, please see the Portfolio Application Status Update Summary for a list of studies submitted to 3CTN for review and approval.
Facilitated Peer Review
The 3CTN Coordinating Centre can help facilitate peer review for an un-reviewed trial. Please see the documents listed below for further details:
- For Sites:
- For Reviewers:

