Executive Message
On behalf of the Network, I am pleased to present our Annual Report featuring highlights and accomplishments from the past year, the 10th since our formation in 2014.
This year has seen sustained rates for new academic cancer clinical trials (ACCTs) added to the 3CTN Portfolio and activated across centres in comparison with the past year. While this signals continued post-pandemic recovery, there has been an observed plateauing of overall recruitment rates, owing in part to trial designs involving more targeted therapies for smaller patient populations. Our capacity to monitor trends such as this in the Canadian trial environment will be invaluable for adapting go-forward accrual strategies and targets.
As post-pandemic recovery of the trial workforce has continued, we are proud to share that notable advancements have been made in leveraging the standardized Joint Task Force for Clinical Trial Competency (JTF) Framework to help our membership of over 200 clinical research personnel self-assess and readily access learning materials relevant to their individual development objectives through our innovative Core Competency Learning Hub. Local trial units have further begun integrating aligned competency-based assessment tools and role descriptions into their operations. Success in Network-wide adoption of the common framework holds significant advantages for helping identify and address knowledge development needs.
Building upon our comprehensive Equity, Diversity and Inclusiveness Framework and thanks to our national funder, the Canadian Partnership Against Cancer, we were able to launch systematic collection of self-reported race data for patients participating in trials on the 3CTN Portfolio at 28 of our member cancer centres. This will, for the first time, enable a clearer, more quantitative view of how trial demographics compare with that of local, regional and national populations. Insights derived from the Network will inform priorities and future strategies for addressing gaps. CPAC funds have also supported cancer centre initiatives designed to create more equitable access to academic cancer trials for underrepresented populations. Projects, including scaled implementation of the widely recognized CRAFT model for decentralized clinical trials (DCT) at adult and pediatric centres, will contribute to a better understanding of best practices needed for sustained improvements.
As you review this year’s report, we invite you to reflect on the unique capacity and essential value that 3CTN’s collaborative networks contribute to Canada’s ACCT ecosystem.

Stephen Sundquist, Executive Director
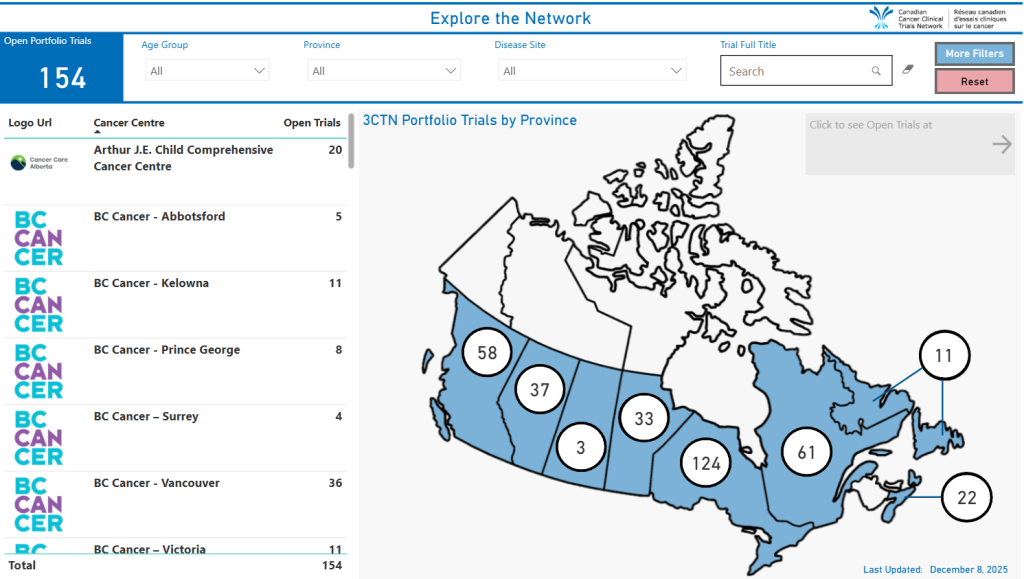
Recruitment
3341 Patients

15 Patients Received Closer to Home Care

60 Adolescent & Young Adult Patients
Advancing equity in cancer clinical trials across Canada
Canadian Partnership Against Cancer advanced 3CTN’s national health equity priorities by funding member cancer centres’ initiatives.
Clinical trials play a vital role in advancing cancer research and providing patients with access to innovative treatments and diagnostic technologies. However, factors such as Canada’s vast geography, cultural, language, and other socio-economic barriers can hinder participation in clinical trials for many patients.
Dedicated funding received this year from the Canadian Partnership Against Cancer (CPAC) has supported six Network sites in implementing equity, diversity and inclusion (EDI) best practices benefitting equity-deserving communities. Sites aimed to build foundational knowledge and identify scalable, practical solutions for including underserved populations in clinical trials. This has enabled sites to develop better understanding of their community members’ needs and priorities, initiate EDI-informed interventions, and inform future program development. Knowledge gained from each initiative will be shared across the Network.
“With 3CTN funding, we’ve partnered with regional teams to establish satellite sites supported by digital tools, bringing early phase trials closer to home, reducing travel demands, and making participation less disruptive for children and families across BC while advancing equity in pediatric cancer research.” – Hina Johnstone, Research Coordinator at BC Children’s Hospital.
Additional CPAC funding is supporting the collection standardized, self-reported race data for patients enrolled in 3CTN Portfolio trials. In total, 28 Network cancer centres will provide baseline information from trial patients that can be compared to demographic makeup of sites’ catchment populations to identify gaps in trial access. Over time, network data can be used to inform targeted strategies for with identified, underrepresented populations to ensure that opportunities to participate cancer clinical trials more equitable across Canada’s diverse population and generate results that contribute to equitable improvements in care.
“We are very thankful to the Canadian Partnership Against Cancer for their continued support. The projects that it will enable represent the next phase in our continued campaign to make access to clinical trials more equitable and make a positive impact on the lives of cancer patients and their families across the country.” – Dr. Janet Dancey, Scientific Director of 3CTN
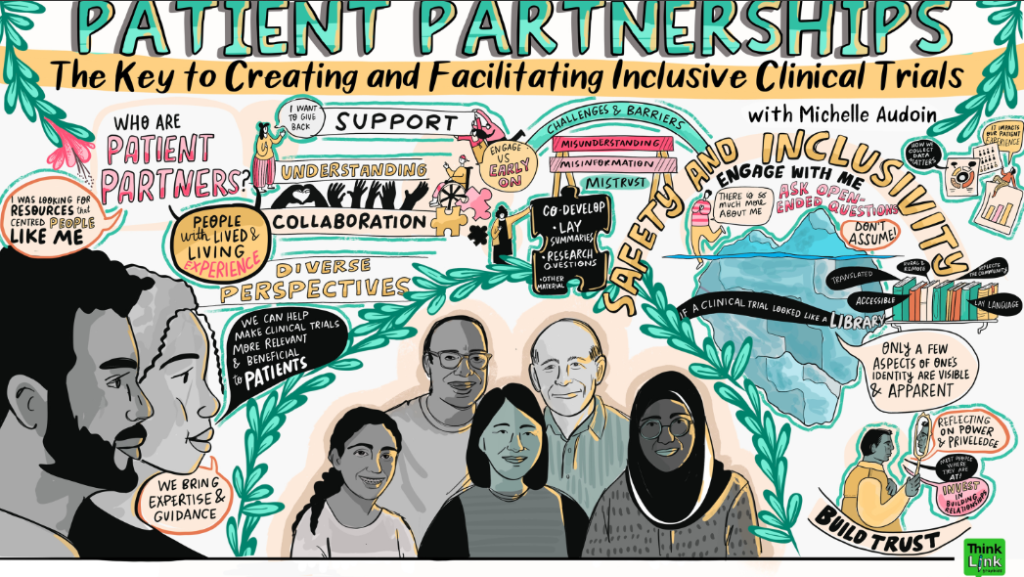

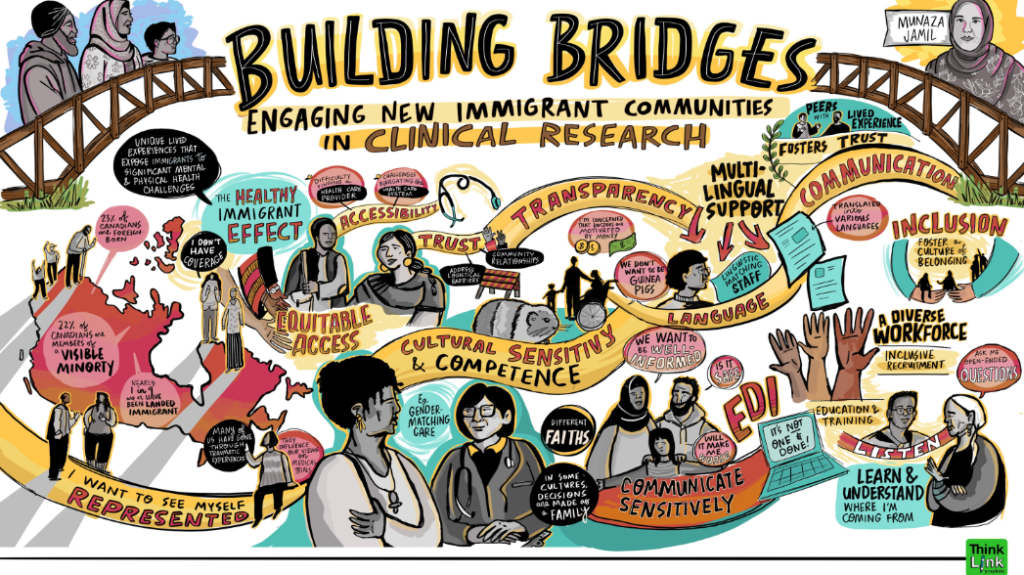
Visual learning summaries by Erica Bota, for the EDI Trials Community UNLearning Platform Series, project led by BC – Cancer Prince George

Bringing trials to patients: advancing decentralized clinical trials in Canada
On March 7, 2025, researchers, patient partners and collaborators from across the country came together for a collaborative workshop focused on advancing the implementation of the Canadian Remote Access Framework for Clinical Trials (CRAFT). The sessions provided updates on the decentralized clinical trials landscape and identified solutions to support broader adoption and scaling of the CRAFT hybrid DCT model. CRAFT continues to be recognized as a pragmatic, feasible approach to expanding equitable trial access and strengthening Canada’s research capacity by better connecting of research and community healthcare centres.
“For patients across rural and remote Canada, CRAFT is a lifeline. A tangible commitment that innovation in cancer care will be accessible to all Canadians, not just those fortunate enough to live near major centres.” – Carla Bossart-Pletzer, Patient Partner and Workshop presenter.
Participants discussed implementation barriers and outcomes and next steps were captured in a report. 3CTN will establish a Steering Committee to provide strategic oversight of CRAFT implementation with related activities included in 3CTN’s workplan through 2027.
Funding for the CRAFT 2.0 Workshop is provided by the Canadian Partnership Against Cancer.
Optimizing patient centered care through continuous process improvements
By Susanna Town, Manager, Clinical Research Unit and Cancer Translational Research Core, Arthur J.E. Child Cancer Centre, Cancer Care Alberta
To meet the growing demand for cancer care trials and to position Arthur Child as a preferred site for trial sponsors, our team launched a focused quality improvement initiative in summer 2024 aiming to reduce trial activation timelines. Since then, our initial focus has been achieving a series of strategic “quick wins,” including mapping out the full activation process to identify bottlenecks and “a-ha moments”, implementing parallel sub-processes for contracts, budgets, pharmacy readiness, and integration of Connect Care (our new provincial Electronic Medical Record). These changes allowed us to streamline workflows and accelerate Site Initiation Visits (SIVs), ultimately offering more timely treatment opportunities to patients.
The Arthur J.E. Child Cancer Centre achieved an average site activation time of 138 days in Y11, which was 107 days faster than their Y10 average, with 4/6 trials activating within the network target of 150 days.
A key factor in our success was fostering a strong, collaborative team environment and breaking down silos that had existed within our unit’s Trial Launch Team. Engaging multiple stakeholders (Legal, Budgets, and Connect Care teams) early in the trial activation process helped align priorities and ensure smoother execution. We also leveraged real-time data tools, such as Microsoft Lists, Power BI and Power Automate to make metrics more accessible and actionable across departments. This data-driven approach enabled us to flag areas for improvement—both immediate and long-term—and respond quickly to evolving needs. Positive feedback from sponsors has reinforced the value of these efforts.
We continue to evaluate our trial activation metrics based on the changes made to date. Work is ongoing to further refine and enhance our processes as we strive for continuous improvements that will allow us to provide the best patient-centered care.

Arthur J.E. Child Cancer Centre, Cancer Care Alberta
Access.
CRAFT Toolkit
Across Canada, access to clinical trials is limited for many patients. Travel time and associated costs for rural and remote populations are prohibitive. With the development and implementation of CRAFT, the Network aims to provide equitable access to clinical trials for all Canadians, regardless of their location.
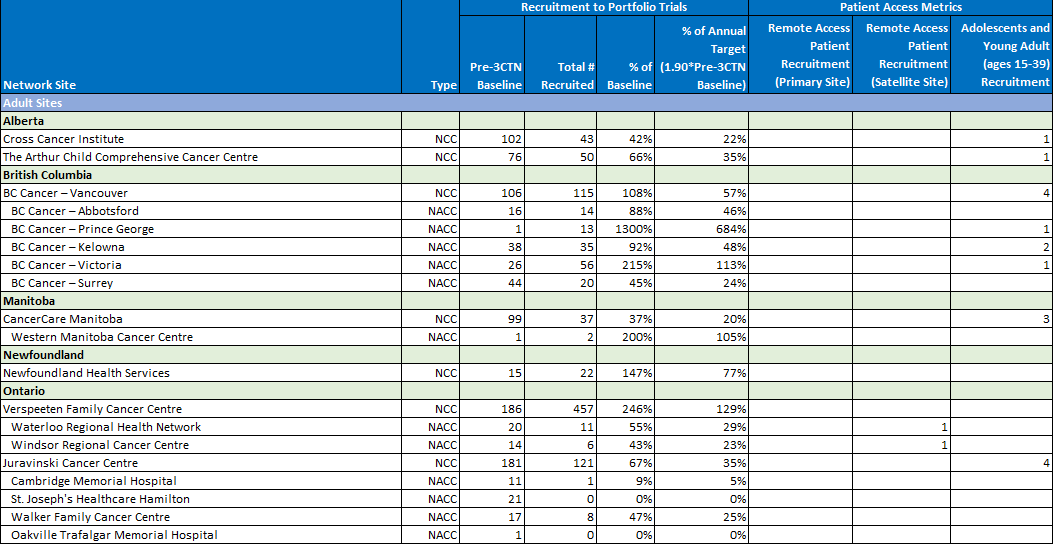
Network Achievements
3CTN made significant progress in improving access for underrepresented trial participants. With additional funding received this year from the Canadian Partnership Against Cancer, key achievements included:
The Network hosted two highly anticipated GAPP Webinar sessions, EMR Implementation: Lessons Learned & Effective Strategies, with separate offerings for Epic and Cerner users. Experienced site research teams led presentations and discussions addressing process and system-integration challenges with attendees from sites currently implementing or planning to implement EMR systems. The webinars aimed to help centres minimize disruptions to trial conduct during the transition periods, and leverage technology to improve the efficiency and quality of trial conduct. Both sessions were well attended and highly rated (Epic: 83 attendees; overall approval rating 4.6/5; Cerner EMR: 53 attendees; overall approval rating 4.7/5).
3CTN, with support from EDGE, University of Southampton, created the Canadian Cancer Trial Finder which consolidated Network data from existing solid tumour trial maps and leveraged an available Application Programming Interface (API) to improve the efficiency and capacity of regular updates from clinicaltrials.gov that has enabled inclusion of all active academic- and industry-sponsored trials at Canadian sites.
| Province | Number of Sites | Selected Network and site highlights |
|---|---|---|
| Alberta | 2 | – Trial staff participated in Diversity and Inclusion learning modules – New process implemented to track completion of staff training – Improved trial activation timelines and successfully activated a trial within 120 days |
| British Columbia | 6 | – Participated in provincial EDI initiatives to improve EDI and best practices for cultural safety – Met local patient recruitment targets – Launched a hospital-wide EMR system – Increased physician engagement and awareness of active trials |
| Manitoba | 2 | – Developed adolescent and young adult (AYA) strategy to pilot with an upcoming clinical trial – Recruited first patient within site target |
| Newfoundland | 1 | – Opened additional Portfolio trials and increased recruitment |
| Ontario | 25 | – Implemented an improved trial activation process, successfully activating a Portfolio trial within 120 days – Implemented a quality management system – Completed root cause analysis to identify factors impacting recruitment – Developed workflow between primary and satellite site for CRAFT implementation |
| Quebec | 2 | – Implemented initiatives to improve communication and collaboration across oncology groups – Connected with site EDI lead to discuss how to improve access to trials |
| Pediatrics | 13 | – Developed an updated workload management tool and piloted across multiple sites using EDGE – Collaborated with adult cancer centre to enroll AYA patients – Implemented an improved trial activation process |
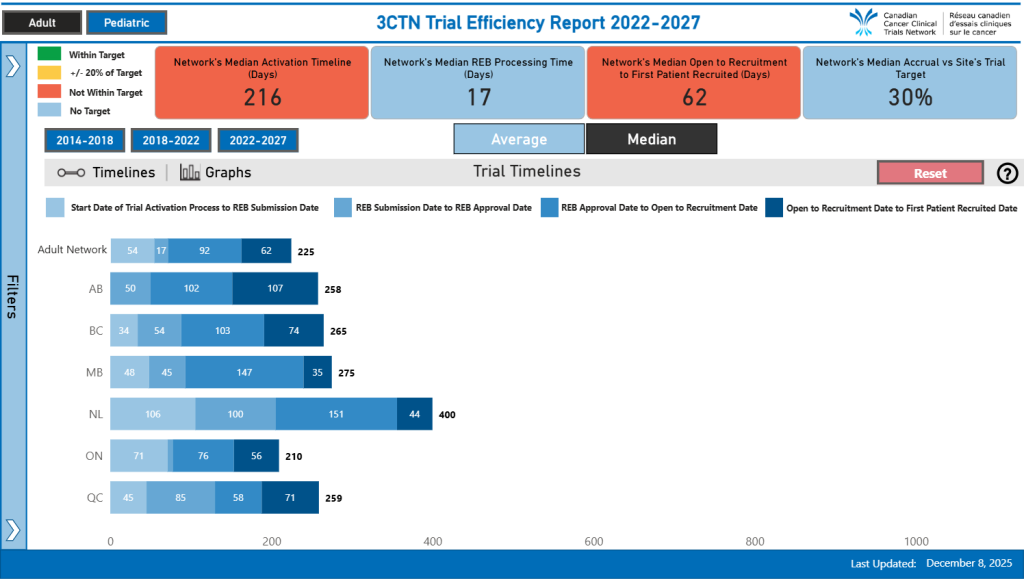
Outcomes.
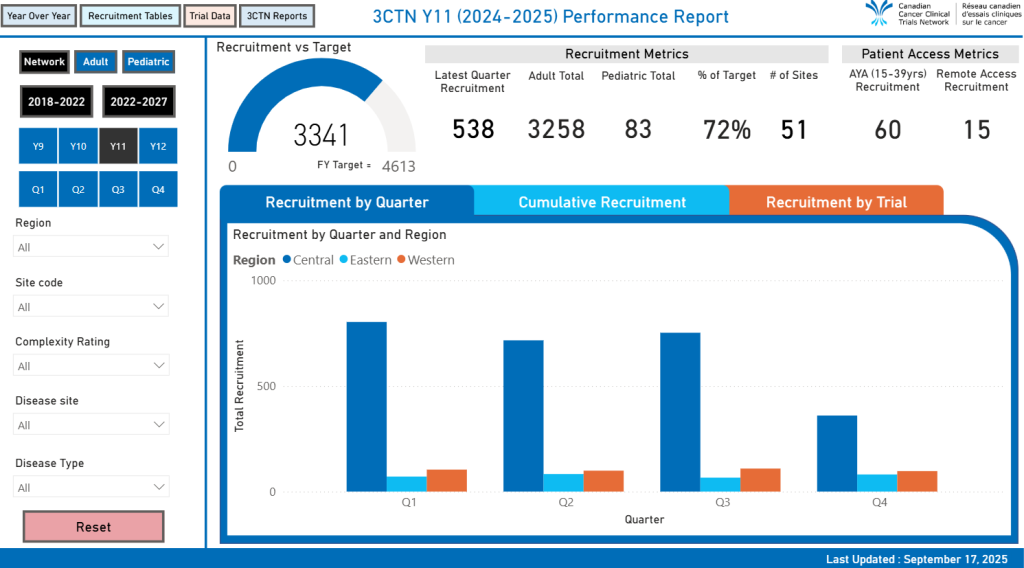
Network Performance Report
Compare and contrast network recruitment over the years. Explore top recruiting trials by quarter and fiscal year. Quickly see the networks performance to annual recruitment targets.
Portfolio Trial Applications

44 Trial Applications Reviewed

3 Facilitated Peer Reviews Completed

44 Trials Approved

3.3 Days Average
0 Days Median
Processing Time
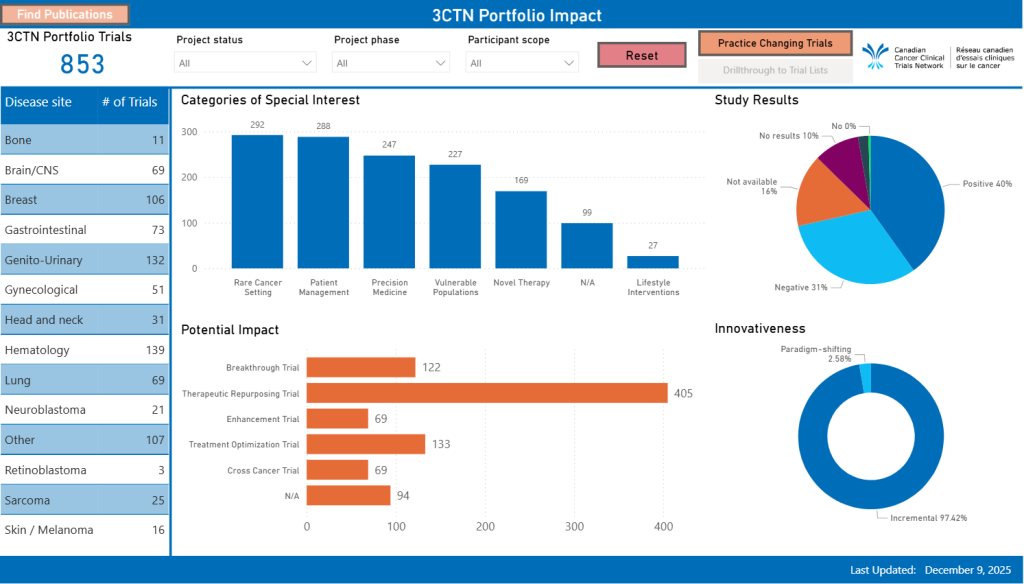
Impact.
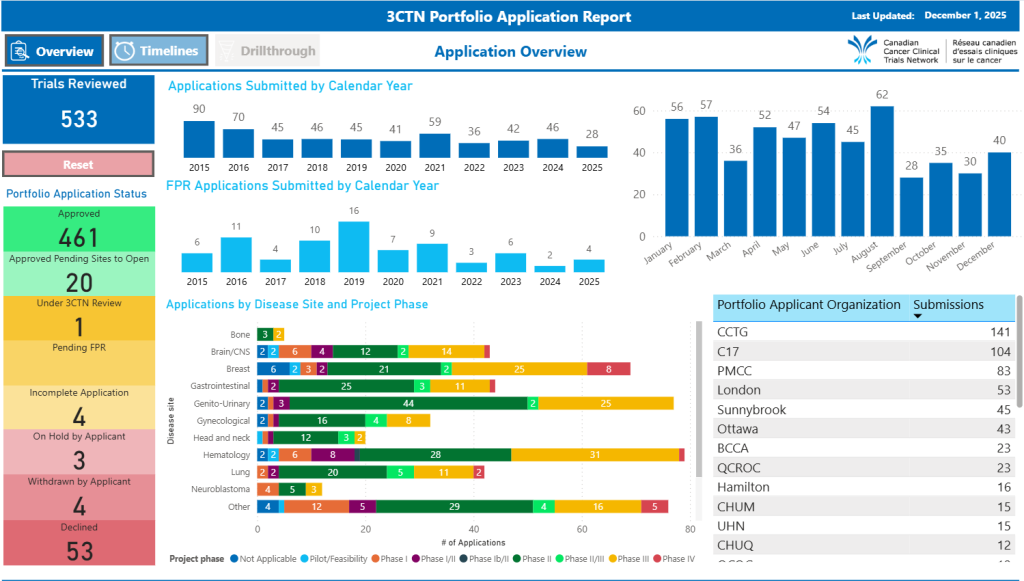
Portfolio Application Report
Compare and contrast 3CTN Portfolio trial applications totals and processing times over the years. See the current status of trials being reviewed. Find out which organizations submit the most Portfolio Applications.
Financials
Revenues
$ Amount in CAD
| National | 2,012,616.63 |
| Provincial | 3,684,099.00 |
| Other | 31,500.00 |
| 5,728,215.63 |
Expenses
$ Amount in CAD
| Network Sites | 4,333,415.74 |
| Coordinating Centre | 416,731.48 |
| Network Costs | 956,605.53 |
| 5,706,752.75 |
Net (revenue less expenses)
$ Amount in CAD
21,462.88